PerPlease Note: As of May 2025, the E-Power Machine is no longer being sold in the USA.
Below is information and downloads for your reference.
The E-Power Machine offers a unique combination of massaging and energizing actions, delivering numerous benefits. It is designed to help alleviate muscular aches, cramps, spasms, and tension. By focusing on enhancing cellular energy, the E-Power Machine promotes improved circulation and energy flow throughout the body’s meridians. This can help relieve soreness and achy joints while supporting a balanced and harmonious functioning of your body systems.
In Traditional Chinese Medicine, optimal health is achieved when energy flows freely through the body’s channels, or meridians. Disease often arises from blockages or disruptions in this energy flow. The E-Power Machine assists in overcoming these blockages by improving the flow of energy, contributing to overall well-being and a more balanced state of health.
What is it?
The E-Power Machine is a form of electrotherapy or Energy therapy. Its premise is somewhat similar to how a PEMF device or PEMF therapy (pulsed electromagnetic therapy) works. Its design provides Negative Potential Energy (Anion Effect) with High-Frequency Energy (Resonance). Your body acts as the capacitor, allowing the E-Power to generate 70 KHz of high-frequency, low-voltage electrical waves, creating an internal cellular energy that balances and revitalizes you.
As the E-Power Machine increases the temperature of your subcutaneous skin, Negative Potential Energy spreads throughout your entire body, relaxing you and balancing the electrical field among your cells. This balances the functions of cations (positively charged ions or positive ions) and anions (negatively charged ions or negative ions) on both sides of the cell membrane. This energy helps recharge your cells, giving you cellular energy, the energy the body needs to function at its best. This promotes a healthier overall metabolism and may help your body systems balance and support a robust immune system.
E-Power’s energy therapy has been used throughout Japan for over 20 years with no side effects. Research has been done at the Convalescent Hospital in China and has been found to benefit those who suffer from Insomnia, Arthritis, and Arthrodynia.
Why Negative Potential is Important
Our bodies are very complex structures, and our fundamental structure is our cells. Cells create the basic structure and function of all living things and have been known for years as life’s building blocks! Every living creature is only as strong or healthy as its cells, and all cells require energy to exist! ATP is the cellular energy and powerhouse of the cell, which is necessary for your overall well-being. Without proper energy, cells can not absorb nutrients and eliminate wastes without a steady cellular energy supply!
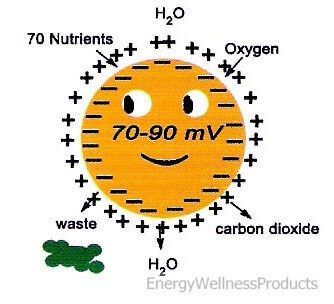
Each cell membrane in our body has a negative electric potential of 30-50 millivolts. When we talk about a cell’s electric potential, we refer to the difference in electric potential between the interior of the cell and the exterior of the cell. Healthy cells have a negative electric potential inside their membrane compared to a more positive electric potential outside of their cell membrane. This difference between positive and negative potential gives our cells the energy they need to function properly. So, considering how many cells we have, which is approximately six trillion, the total electrical potential of the whole human body is huge!
Negative Potential of our Cells
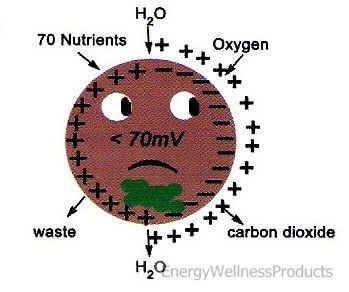
Our negative potential energy is approximately 70-90 millivolts when we are young. When we become sick or tired, our negative potential energy becomes lower than 60 millivolts. As we age, our negative potential energy decreases, and this reduction may and often does cause illnesses. When our negative potential energy hits zero, we are no longer living. For this reason, it is necessary to increase our electrical potential to improve our health. Living in today’s environment, under constant stress, fighting off viruses and bacteria, and aging, the E-Power offers us the tool we need to keep the body’s cellular energy up and functioning at the optimal level so we can stay healthy!
Energizing
The E-Power Machine energizes our body by giving our cells negative potential energy. This energy helps activate our cells and breaks through traditional treatments to support the functions of the body’s eight main systems and energy flow through the meridians. The E-Power is safe and can be used by people of all ages, from infants to those old enough to retire.
It is said that about 80% of illnesses in the human body stem from the circulatory system. A Cardiology Specialist from the United States ( W. Casteli) says, “Good maintenance of the heart and the circulatory system can prolong life.” E-Power helps you keep your circulatory system healthy!
Negative Potential Energy = Energy Needed for Rest
High Frequency Energy = Energy Needed for Exercise (ATP)
Negative Potential Energy is also needed to increase the permeability of cell membranes and enhance the composition of ATP (adenosine triphosphate – energy-carrying molecules found in the cells of all living things). The enzyme ATP is responsible for transporting chemical energy within our cells for our metabolism. ATP is a complex molecule that stores and supplies the cellular energy the body needs. The E-Power gives this energy to our cells so they can function at their best, taking in important nutrients and oxygen and getting rid of wastes such as toxins and carbon dioxide! Our bodies prefer ATP over any other form of energy, including fat!
An individual gave me an example of creating negative potential energy in the body: “When someone dies in a hospital and they give them those two paddles, they are actually giving them a high voltage of negative potential energy, which jump-starts every cell in their body. The E-Power on the high setting generates 1,000 volts of negative potential so we can slowly recharge every cell in our body, improving overall cellular energy and energy flow.”
The Difference between Negative Potential and Positive Potential Energy
If you have too much positive potential energy around the cell membrane, the cell cannot absorb or take in nutrients, oxygen, and water, and get rid of wastes and carbon dioxide. The elevation of positive potential energy results in the cell receiving too much sodium and hydrogen. On the other hand, when the negative electric potential inside the cell is greater than the positive potential outside the cell, nutrients easily enter the cell, and wastes quickly exit the cell. The ease of this exchange is largely due to the continual exchange of sodium and potassium from inside the cell to outside of the cell, opening up the cell’s membrane.
E-Power Machine Benefits

- Balances Cellular Energy and the Nervous system
- Activates cells and helps promote oxygen intake (relaxation)
- Promotes Circulation
- Helps detoxify the body
- Improves immune system performance
- Improves skin – Facial Skin Rejuvenation
- Assists in weight loss
- Increases ATP (produces energy)
- Helps reduce stress
- Improves the quality of sleep
- Promotes the flow of Chi through the Meridians
- Promotes pH Balance
- Facial Skin Rejuvenation
Features of the E-Power Machine

- Easy to Handle and Use | FAQ and How to Use” the E-Power Machine.
- Portable & Convenient
- No Known Negative Side Effects
- Internationally Patented Design
- Noticeable Results
- Two Ports for Two People to Use
- Comes with One E-Power Belt and One Indicator Pen.
FAQ on the E-Power Machine
1. What is a PEMF device, and how does it work? Als,o is it the same as the E-Power Machine?
A PEMF (Pulsed Electromagnetic Field) device is a therapeutic tool that uses low-frequency electrical currents to generate a magnetic field to stimulate and energize cells in the body. By emitting pulses of electromagnetic energy, the device aims to improve cellular function, enhance circulation, and support overall wellness. The electromagnetic fields penetrate the body’s tissues, promoting natural healing processes and cellular rejuvenation. Although the E-Power offers similar benefits to a PEMF Device, instead of creating electrical currents, it creates an e-field that provides negative potential energy to penetrate the body’s tissues.
2. How does The E-Power support cellular energy and overall well-being?
The E-Power supports cellular energy by enhancing the body’s ability to regenerate and repair itself at the cellular level. The negative potential energy helps the cell’s membrane permeability, which facilitates the exchange of nutrients and waste products. This stimulation helps to increase cellular energy (ATP production), reduce inflammation, and promote better circulation, contributing to overall well-being and vitality.
The Energy Wand
The Energy Wand is used to further open up the flow of energy through the body. It can be used to target trouble areas where you are searching for relief from aches, pain, and inflammation. Or it can be used to promote the flow of energy through the twelve meridians of the body. In Traditional Chinese Medicine, the proper flow of energy or chi is necessary to maintain optimal health, and the Energy Wand is your tool for maximizing this flow.
In TCM, their belief is that these 12 meridians of the body correlate to organs, joints, and extremities. The 12 meridians of the body are the lung, pericardium, heart, small intestine, triple heater, large intestine, stomach, gall bladder, bladder, spleen, liver, and kidney. Utilize the power of this energy therapy with the Energy Wand to optimize overall health and wellness.
E-Power Facial Skin Rejuvenation
E-Power is amazing for facial skin rejuvenation, one of my favorite benefits from this type of energy therapy. I find that using a Facial Mask during a 30-minute session on medium with the E-Power is an extra benefit that tightens, tones, and improves my skin. It is similar to a high-frequency facial treatment. I feel that it also demonstrates the power of the E-Power Machine and its effect on the body overall!
Product Specifications and Precautions
- Negative Potential / High Frequency Wave Health Care Instrument
- Model # HBM-618
- Weight: 6.61 lbs
- Size: 11″ x 10.55″ x 5.55″
- Waist Belt: 49.6″ x 5.9 inches
- AC Voltage – USA 90- 120V
- Input Power – 18 Watts + 20%
- Power Supply Frequency – 50- 60Hz
- High Peripheral Wave Frequency – 70KHz +/- 10%
- Timer: 0, 30, 60, 90 minutes and a Continuous Mode which runs for 3 Hours
- Three operating modes
- High Peripheral Wave: 2500 +/- 100V; Middle: 2300 +/- 100V; Low: 1900+/- 100V
- Negative Electrical Potential : High: -1000 +/- 100V; Middle: -800 +/- 100V; Low: -600 +/- 100V
Precautions: This should not be used if you have a pacemaker, if you are menstruating, if you are pregnant, if you have a high fever or tumors, with metallic items such as badges or jewelry, if you have implants of gel or silicon and transplant organs, if you have a history of Heart Problems, or if you drink alcohol within one hour of each use. If you have a medical condition and have a question, you should consult with your physician before using.
Downloads on the E-Power Machine
- E-Power Brochure
- Guide and Instructions for the E-Power
- Research Article on the E-Power
- E-Power Testimonials
- Doing an in-home Facial with the E-Power
- Opening up the Meridians with the Energy Wand
- Using the Wand for Acupuncture Massage
Recommendations on Products
It isn’t easy to express interest in another product. However, the NeoRhytym Omni PEMF offers a variety of devices as well as mats offering PEMF technology for healing. These devices have helped many of my clients with difficulty sleeping, improving and balancing mood, and managing pain. As an affiliate, you can check them out via my link.
